Ozone CT Value Explained
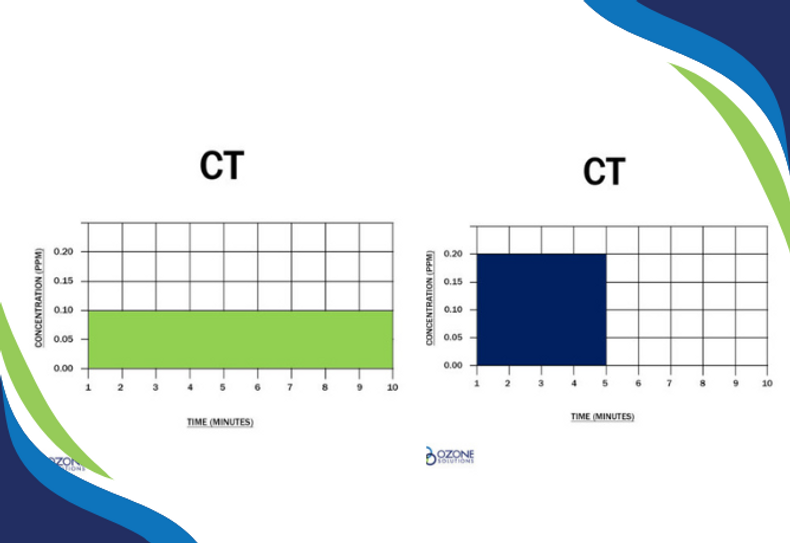
"CT" is the product of "residual disinfectant concentration" (C) in mg/l and the corresponding "disinfectant contact time" (T) in minutes. In other words, ozone CT is the dissolved ozone concentration multiplied by the contact time (remember that 1 mg/l = 1 ppm.)
Some sanitizing treatments with ozone can be accomplished very quickly, but some treatments require sufficient ozone in the water along with an adequate contact time. This contact time is required for the dissolved ozone to oxidize organic contaminants and to disinfect the water.
This CT value is assumed to be unitless. Either the concentration can be increased while the time is decreased or vice-versa, to assure a given level of disinfection is obtained.
For example, a CT value the bottled water industry generally uses is 1.6. This means the dosage rate is 1.6 mg/l minutes. The operator could ozonate at 0.2 ppm for 8 minutes or at 0.4 ppm for 4 minutes. Either would work as long as the final CT is 1.6.
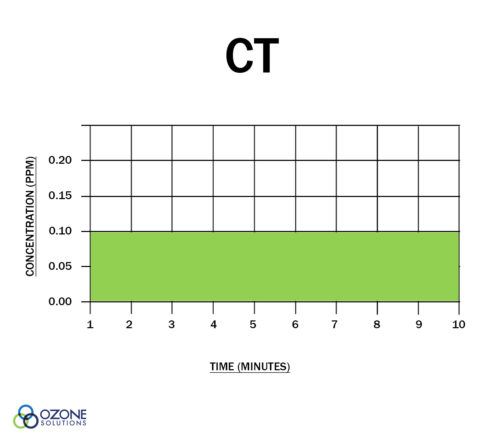
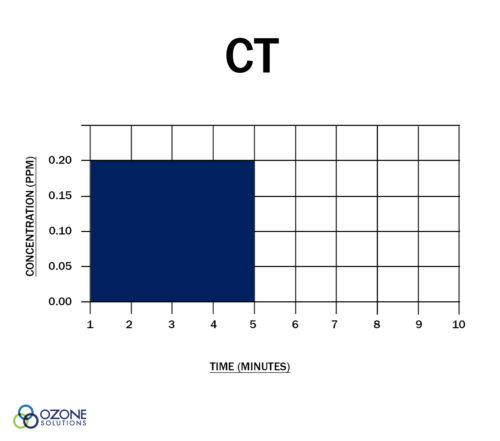
Both Charts Show a CT Value of 1.0
You may have heard the claim, "ozone is 3,000x more germicidal than chlorine." What does this mean? This statement hinges on the fact that for some organisms, you need a CT value 3,000x higher when using chlorine vs. ozone. Put another way, if a dissolved ozone level of 0.2 ppm for 1 minute (CT is 0.2) is needed to inactivate a specific microorganism, you would need 200 ppm of chlorine for 3 minutes (CT = 600) to achieve the same kill effect.