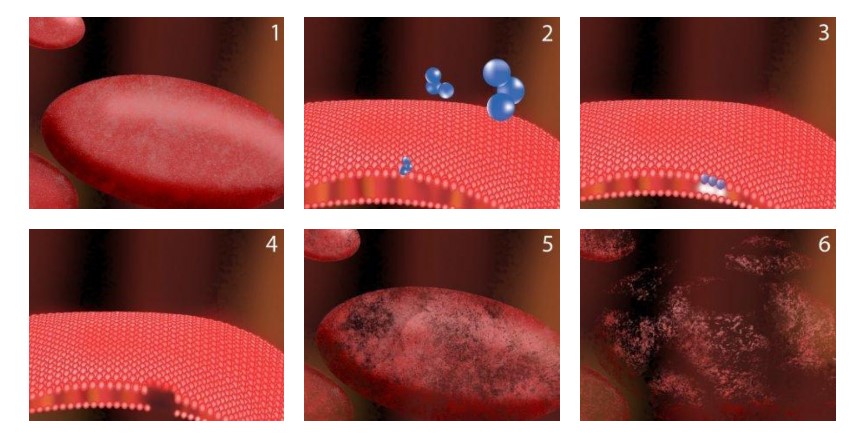
The third oxygen atom of ozone is extremely reactive because it is unstable. This atom readily attaches itself to other odor molecules. When contaminants such as odors, bacteria, or viruses make contact with ozone, their chemical structure is changed to less odorous compounds. As more ozone attacks the remaining compounds, the odor is eventually destroyed. This process is called oxidation. Ozone essentially reverts to oxygen after it is used. This makes it a very environmentally friendly oxidant.
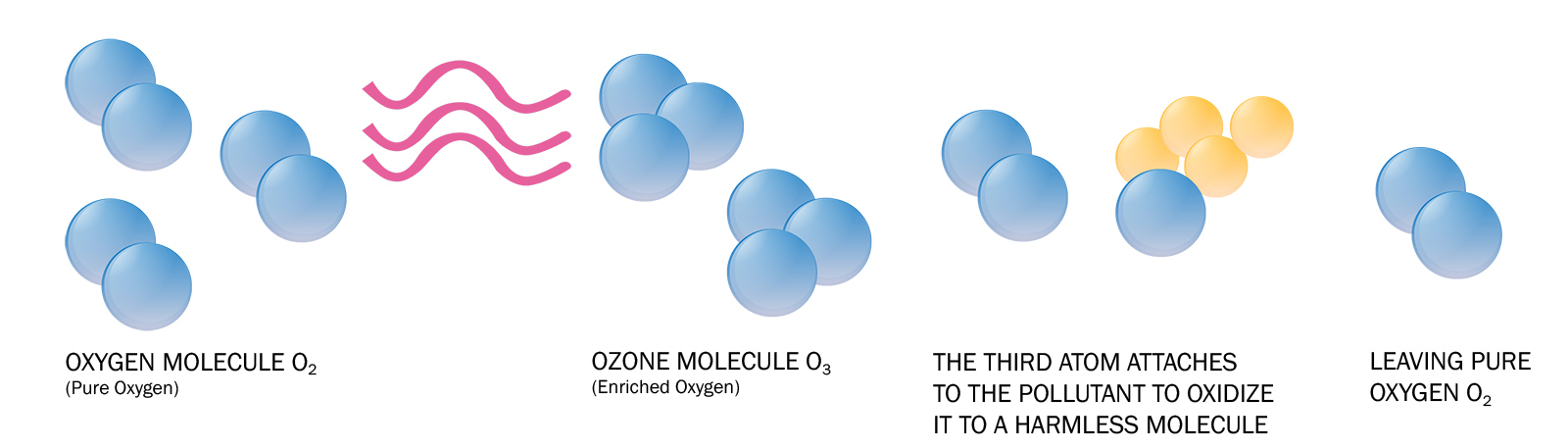
Ozone not only destroys bacteria cells, but it does it without leaving a residual effect. See the effects ozone has on bacteria in the 6 steps below.