Ozone vs. Chlorine for Water Disinfection
Across the U.S., municipal water treatment agencies are faced with evaluating ozone systems for water treatment to help safeguard against bacteria, viruses, and pathogens. According to the EPA, “Disinfection is considered to be the primary mechanism for the inactivation of pathogenic organisms to prevent the spread of waterborne diseases to downstream users and the environment.” (See Table 1 for some common micro-organisms found in domestic wastewater and the diseases associated with them.)
TABLE 1: Infectious Agents Potentially Present in Untreated Domestic Wastewater
| ORGANISM | DISEASE CAUSED |
|---|---|
| BACTERIA | |
| Escherichia coli (enterotoxigenic) | Gastroenteritis |
| Leptospira (spp.) | Leptospirosis |
| Salmonella typhi | Typhoid Fever |
| Salmonella (=2,100 serotypes) | Salmonellosis |
| Shigella (4 spp.) | Shigellosis (bacillary dysentery) |
| Vibrio cholerae | Cholera |
| PROTOZOA | |
| Balantidium coli | Balantidiasis |
| Cryptosporidium parvum | Cryptosporidiosis |
| Entamoeba histolytica | Amebiasis (amoebic dysentery) |
| Giarda lamblia | Giardiasis |
| HELMINTHS | |
| Ascaris lumbricoides | Ascariasis |
| T. solium | Taeniasis |
| Trichuris trichiura | Trichuriasis |
| VIRUSES | |
| Enteroviruses (72 types, e.g., polio, echo, coxsackie) | gastroenteritis, heart anomalies, meningitis |
| Hepatitis A | Infectious hepatitis |
| Norwalk agent | Gastroenteritis |
| Rotavirus | Gastroenteritis |
Source: Adapted from Crites and Tchobanoglous, 1998
Both ozone and chlorine can be used to treat these organisms. So, the question when it comes to comparing ozone vs. chlorine for water disinfection is, “Which is better?” Chlorine has long been the default choice. It is a powerful oxidizer and is very effective at treating many pathogens. However, factors such as environmental issues, long-term cost, particulate removal effectiveness, and health issues have made ozone the preferred choice for many municipalities.
Ozonated Water Disinfects Better than Chlorine
Ozone, like chlorine, is an oxidizing agent effective at eliminating bacteria in water. It is recognized as among the strongest and fastest commercially available disinfectants and oxidants for water treatment. While chlorine does kill many microorganisms, it cannot treat all water-borne pathogens if used at EPA-approved doses. On the contrary, ozone can remain well within EPA regulations and be effective. When ozone decomposes in water, the free radicals formed, hydroperoxyl (HO2) and hydroxyl (OH), have great oxidizing capacity and play an active role in disinfection. Bacteria are destroyed by protoplasmic oxidation, which results in cell wall disintegration (cell lysis).
The Ozonation Process
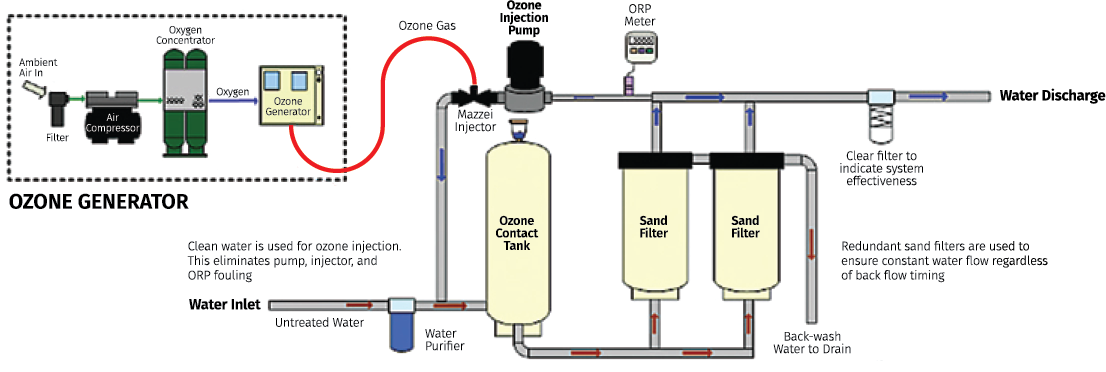
Ozone is created using an ozone generator. This exposes a stream of air to either UV light or to a high voltage electrical discharge, the method known as corona discharge. The corona discharge method is typically preferred and takes place by breaking apart the two oxygen atoms of an oxygen molecule (O2) and then charging one of those atoms to another oxygen molecule, thus creating ozone (O3) molecules. Unfortunately, ozone cannot be stored or packaged because of its instability. Its effectiveness will depend on CT, which will be explained later.
In a water treatment application, the raw water passes through a venturi injector, which creates a vacuum and pulls the ozone gas into the water. An alternate method is bubble diffusion, where air bubbles up through the water being treated. Since the ozone will react with other contaminants or metals to create insoluble metal oxides, post filtration is sometimes required.
Ozone CT Explained
CT is the product of residual disinfectant concentration (C) in mg/l and the corresponding disinfectant contact time (T) in minutes. In other words, ozone CT is the dissolved ozone concentration multiplied by the contact time.
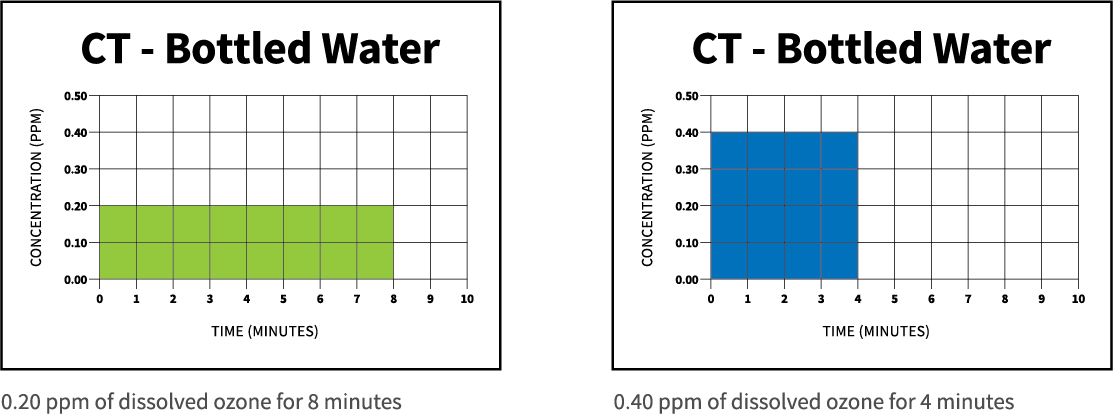
Some sanitizing treatments with ozone can be accomplished very quickly, but other treatments will require higher levels of ozone or longer contact time in the water. This contact time is required for the dissolved ozone to oxidize organic contaminants and disinfect the water. This CT value is assumed to be unitless. To ensure a given level of disinfection is obtained, either the ozone concentration can be held constant while the time is varied or vice-versa. For example, the CT value the bottled water industry generally uses is 1.6. This means the dosage rate is 1.6 mg/l minutes. Ozonation can happen at 0.2 ppm for 8 minutes or 0.4 ppm for 4 minutes. Either approach yields a final CT of 1.6.
The CT value is roughly 3000x higher when using chlorine compared to ozone. For example, if a dissolved ozone level of 0.2 ppm for 1 minute (CT=0.2) is needed to inactivate a specific microorganism, you will need 200 ppm of chlorine for 3 minutes (CT=600).
How Bad Is Chlorine?
Chlorine is a powerful, cost-effective disinfecting agent for many applications. It has played a transformative role in bringing clean, safe drinking water to many areas throughout the world. But we are just beginning to assess the overall health impact of chlorine as a disinfectant in our water.
There is an abundance of well-founded concern about chlorine. Studies have shown that when chlorine is added to our water, it combines with other natural compounds to form Trihalomethanes (chlorination by-products), or THMs. These chlorine by-products trigger the production of free radicals in the body, causing cell damage, and they are highly carcinogenic. “Although concentrations of these carcinogens (THMs) are low, it is precisely these low levels that cancer scientists believe are responsible forthe majority of human cancers. Breast cancer, which now affects one in every eight women in North America, has recently been linked to the accumulation of chlorine compounds in the breast tissue". A study carried out in Hartford, Connecticut, found that “women with breast cancer have 50% to 60% higher levels of organochlorines (chlorination by-products) in their breast tissue than women without breast cancer.”
This increased link to cancer-causing compounds is corroborated in another recent study. According to Johns Hopkins University, “Toxic and carcinogenic compounds are produced when phenols in drinking water mix with chlorine.”
However, it’s not just the threat of cancer that is problematic when evaluating chlorine. A study by the American Chemical Society showed that chlorine use in water and sewage treatment may be contributing to antibiotic resistance. Beyond direct human impact, there are potential environmental impacts to microorganisms that may pose little health threat to humans but play a vital role in various ecosystems.
When evaluating this data, chlorine has meaningful risks. But what are ozone's other specific advantages?
Advantages of Choosing Ozone vs. Chlorine
- Health Benefits: Chlorine can form chloroforms, linked to cancer, while ozone leaves no harmful by-products and reverts to oxygen if unused.
- Effectiveness: Ozone is a stronger and faster disinfectant, purifying water 3000 times faster than chlorine. It can treat all water-borne pathogens, including those resistant to chlorine, like protozoa, E. coli, and Giardia.
- Convenience: Ozone is generated on-site, eliminating the need for storage and transportation.
- Mineral Removal: Ozone helps remove iron, manganese, and sulfur minerals.
- No Residual Taste or Smell: Ozone-treated water has no chemical taste or smell.
- Gentle on Skin: Low-dose dissolved ozone in water does not irritate skin, nose, or ears.
- Efficiency: No rinse steps are needed, saving time and water costs.
- Corrosion: Ozone is less corrosive than chlorine, especially compared to salt chlorination.
- pH Independence: Ozone is effective across a wide pH range and does not affect the water’s pH.
- For long-term waterborne disinfection requirements, ozone is clearly the safer, more environmentally friendly, and lower-cost option. If you have any questions about how ozone can provide better results for your needs, please get in touch with us today.
- #AqueousOzone #OzoneInWasteWater #OzoneVsChlorine #WasteWater #WasteWaterDisinfection #Water #WaterDisinfection
