How Does Ozone Work?
Because of the way that ozone reverts back to oxygen over time, ozone cannot be delivered in tanks. Instead, ozone must be produced on-site by an ozone generator.
Ozone Generators
There are basically two methods of producing ozone:
- Corona Discharge
- Ultra-Violet Light

Corona discharge creates ozone by applying high voltage to a metallic grid sandwiched between two dielectrics. The high voltage jumps through the dielectric to a grounded screen and in the process, creates ozone from oxygen present in the chamber. This also occurs naturally during lightning storms.
Ultra-violet (UV) light creates ozone when a wavelength at approximately 185 nm (nanometers) hits an oxygen atom. The molecule (O2) splits into two atoms (O) which combine with another oxygen molecule (O2) to form ozone (O3.) This also occurs naturally through the sun rays.
Ozone generators produce ozone by adding energy to oxygen molecules (O2,) which cause the oxygen atoms to part ways and temporarily recombine with other O2 molecules, creating ozone (O3.)
Ozone in Action
Once ozone is produced it reacts with a pollutant, often long-chain carbon (organic) molecules, and breaks it down into less complex (and typically less harmful) molecules through a process called oxidation.
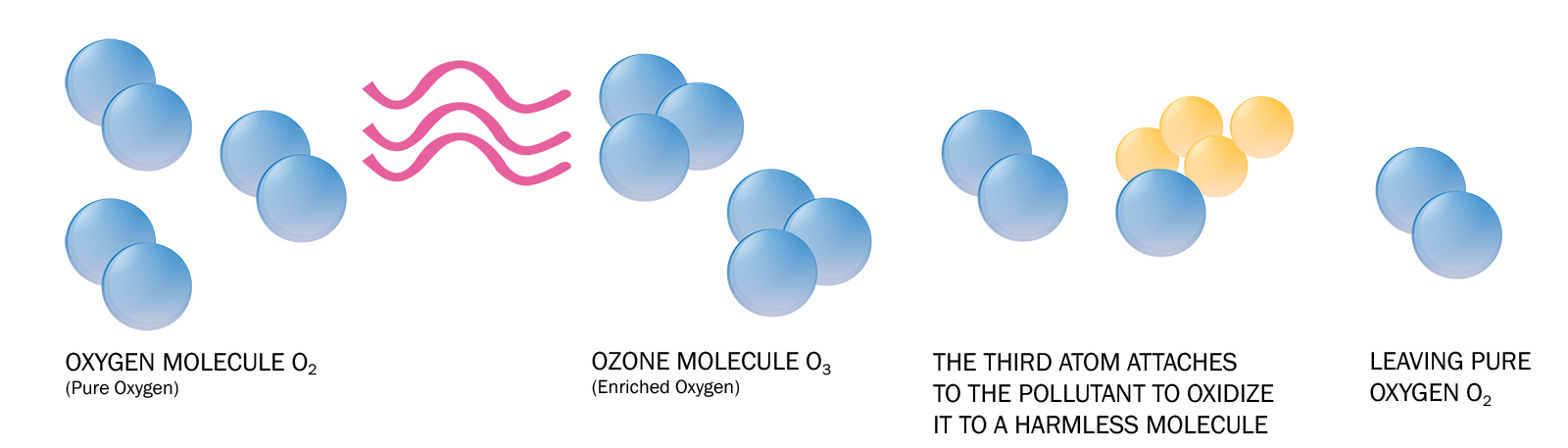
Ozone's Lifespan
As soon as ozone is formed in a generator and dispersed in a room, some of it reverts back into oxygen (O2.) This step occurs by several processes including the following:
Oxidation reacts with an organic material such as odors or smoke. Reactions with bacteria, etc., which again consumes ozone by oxidation reactions.
Additionally ozone breaks down thermally. Higher temperatures destroy ozone quicker than lower temperatures. The ozone that remains is referred to as Residual ozone. "Residual" ozone created will return to oxygen usually within 30 minutes, in amounts equal to half its level. This is called the half-life. In other words, every 30 minutes half of the ozone in the room will be left; the other half will have reverted back into oxygen.
Therefore, ozone - while very powerful - does not last long. It does its job and then disappears into safe oxygen.
Ozone is one of the most powerful oxidants used in water and air applications.
See the different properties of ozone below:
| PROPERTY | OZONE | OXYGEN |
|---|---|---|
| Color | Light Blue | Colorless |
| Smell | Smell after Lightning Storm | Odorless |
| Density (g/l) | 2.144 | 1.429 |
| Molecular Weight | 48 | 32 |
| Solubility in Water (0° C) | 0.640 | 0.049 |
| Electochemical Potential (volts) | 2.070 | 1.230 |
Typical O3 Half-Life vs. Temperature
| GASEOUS TEMP (°C) | GASEOUS HALF-LIFE* | DISSOLVED TEMP (°C) | DISSOLVED HALF-LIFE |
|---|---|---|---|
| -50 | 3 Months | 15 | 30 Minutes |
| -35 | 18 Days | 20 | 20 Minutes |
| -25 | 8 Days | 25 | 15 Minutes |
| 20 | 3 Days | 30 | 12 Minutes |
| 120 | 1.5 Hours | 35 | 8 Minutes |
| 250 | 1.5 seconds |
* These values are based on thermal decomposition only. No wall effects, humidity, organic loading or other catalytic effects are considered.
Ozone Solubility
The solubility of ozone depends on the water temperature and the ozone concentration in the gas phase: Units in g/m3.
| OZONE GAS | 0°C | 5°C | 10°C | 15°C | 20°C | 25°C | 30°C | 35°C |
|---|---|---|---|---|---|---|---|---|
| 25 g/m3 | 16.00 | 12.50 | 9.75 | 7.75 | 6.00 | 4.75 | 3.75 | 3.00 |
| 50 g/m3 | 32.00 | 25.00 | 19.50 | 15.50 | 12.00 | 9.50 | 7.50 | 6.00 |
| 75 g/m3 | 48.00 | 37.50 | 29.25 | 23.25 | 18.00 | 14.25 | 11.25 | 9.00 |
| 100 g/m3 | 64.00 | 50.00 | 39.00 | 31.00 | 24.00 | 19.00 | 15.00 | 12.00 |
| 125 g/m3 | 80.00 | 62.50 | 48.75 | 38.75 | 30.00 | 23.75 | 18.75 | 15.00 |
| 150 g/m3 | 96.00 | 75.00 | 58.50 | 46.50 | 36.00 | 28.50 | 22.50 | 18.75 |
| 175 g/m3 | 112.00 | 87.50 | 68.25 | 45.25 | 42.00 | 33.25 | 26.25 | 21.00 |
| 200 g/m3 | 128.00 | 100.00 | 78.00 | 62.00 | 48.00 | 38.00 | 30.00 | 24.00 |
Note: 14.3 g/m3 = 1% ozone
Example: 50 g/m3 = 3.5% ozone
Safety
Ozone is attractive as an alternative to chemical processes like chlorine, which presents significant safety challenges. Low-level exposure to ozone has not been shown to present long-term health problems. In fact, the USDA and FDA have approved ozone for use with food meant for human consumption.
However, there are several governmental regulations regarding the use of ozone in a workplace. For example:
- OSHA requires that ozone levels around workers remain below 0.1 ppm
- OSHA requires that an ambient ozone monitor be in use for generators that produce more than 5 g/hr
The USDA and FDA regulate how ozone may be used in food. In any ozone implementation where humans are present, it is important to make sure there is proper ventilation and/or ozone destruction.
Other Resources
